Ch. 4 Covalent Compounds, Formulas, Structures
Lewis Structures
Covalent Bonds: Electrons are shared, physically attached to each other in molecules
Lewis electron-dot structures used to represent sharing
one electron shared from each atom one covalent bond
two/three pairs shared double/triple bond
octet rule: noble-gas configuration achieved when Lewis structure shows eight electrons around each atom- except , only requiring two
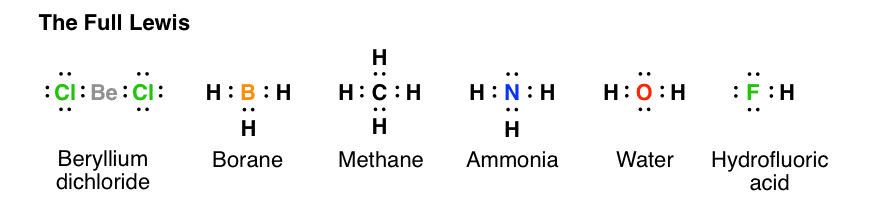
- except , only requiring two
unpaired electrons generate magnetic field make substance paramagnetic
all electrons paired, cancelling most magnetic field make substance diamagnetic, small magnetic field
Drawing Lewis Structures
- Determine central atom
I. atom(s) are usually central
II. is never central
III. Halogens are usually not
IV. Oxygen is usually not, though may link
V. atom appearing only once may be central - Put atoms/groups around central atom to form the skeleton
- Find the total number of valence electrons (take into account charge if ion)
- Add single bonds between each ion
- Fill octets on outer atoms and put remaining in central (nonbonding or lone pairs)
- Change some outer atom lone pairs to double bonds if central atom does not have a full octet unless it is (may have more, if period 3-7)
- Check formal charges
- Some structures may have multiple possibilities, called resonance structures
- actual molecule is like a hybrid of resonance structures, imagine "1.5 bonds"
- Some structures have odd number of electrons free radicals
- may form dimers to pair them, e.g.
Formal Charge
- technique to analytically determine if a Lewis structure is reasonable
- For each atom, count all nonbonding electrons
- Count half the bonding electrons (e.g. one bond counts as 1 electron)
- Add the counts from step 1 and 2
- Compare to number of valence electrons
- low formal charges are generally better
- atoms with higher electronegativity generally have more negative formal charge
left is better because of all 0 compared to a -1 and +1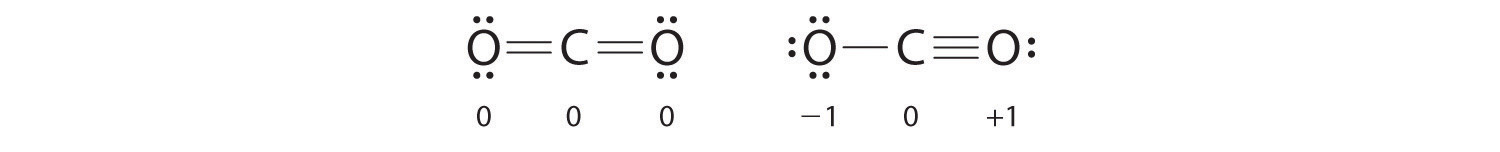
Covalent Bond Polarity
- based on electronegativity difference
- polar if different, range from slightly polar to very polar
- nonpolar if no difference
Dipole Moments
where is the difference in charge (polar molecules will have a slightly negative and slightly positive atom) and is the distance in meters
- unit is debye, where
Ionic Character
- largest is at
- is 50% ionic and 50% covalent in character
difference in electronegativity
| Ionic Character | |
|---|---|
| Nonpolar Covalent | |
| Polar Covalent | |
| Ionic |
Characteristics of Bonds
Bond Order
- average number of bonds an atom makes
- single bond is 1, double bond is 2, triple bond is 3
- in where there are 4 bonds and 3 atoms, the bond order is
Bond Strength/Energy
- triple > double > single in bond strength
- bonded atoms vibrate, and the frequency is related to bond energy (strength) from
- low energy molecules release more energy upon combustion
Bond Length
- in general, single > double > triple
Nomenclature
- include numeric prefix (mono, di, tri, etc.) (except first atom never gets mono), end second with -ide
- e.g. is dinitrogen trioxide, is nitrogen monoxide
Molecular Geometry
- VSEPR theory (valence-shell electron-pair repulsion): negatively charged electron pairs repel each other as far apart as possible
- bonding electron pairs occupy bonding domain
- nonbonding electron pairs occupy nonbonding domain
Molecular Geometry Table
is central atom, and are bonding and nonbonding pairs
| Notation | Shape | Example | Angle |
|---|---|---|---|
| Linear | |||
| Linear | |||
| Linear | |||
| Planar Triangle | |||
| Bent | |||
| Tetrahedron | |||
| Triangular Pyramid | |||
| Bent | |||
| Trigonal Bipyramid | , | ||
| Distorted Tetrahedron | , | ||
| T-shape | |||
| Linear | |||
| Octahedron | |||
| Square Pyramid | |||
| Square Planar |
Molecular Polarity
- symmetrical molecules are nonpolar
- nonsymmetrical molecules are polar if bonds are polar
- molecule with >1 type of atom attached to the central atom is often nonsymmetrical, so is polar
- central atom with nonbonding electron pairs are often nonsymmetrical, so is polar
- one end is positive, other is negative
Covalent Bond Formation
- valence bond theory (VB theory): paired electron spins overlaps atomic orbitals, creating covalent bond
- molecular orbital theory (MO theory): molecule also has distinct energy levels with electrons
- results are the same
- an overlap between 2 s orbitals, an s orbital and a p orbital, or 2 p orbitals form sigma bond ()
- every bond has only 1 sigma bond
- sideways overlap of 2 p orbitals forms a pi bond ()
- can be 0, 1, or 2 to make single, double, or triple bonds in combination with the sigma bond
- In short:
- single bond:
- double bond:
- triple bond:
Hybridization
- Hybrid orbitals explain complex geometries and covalent bonds
- basically count the number of bonds and lone pairs on the central atom
- 2 total: sp
- 3 total: sp
- 4 total: sp